What You Will Learn
After reading this note, you should be able to...
- This content is not available yet.
Introduction
Definition
According to WHO, anemia in pregnancy is present when the hemoglobin concentration in peripheral blood is 11g/dl or less.
However for our environment a hemoglobin concentration of 10g/dl is generally accepted as anemia in pregnancy.
note
Severity of anemia (PCV)
- Severe anemia is ≤ 18%
- Moderate is 19-24%
- Mild is 25-29%
Physiological changes
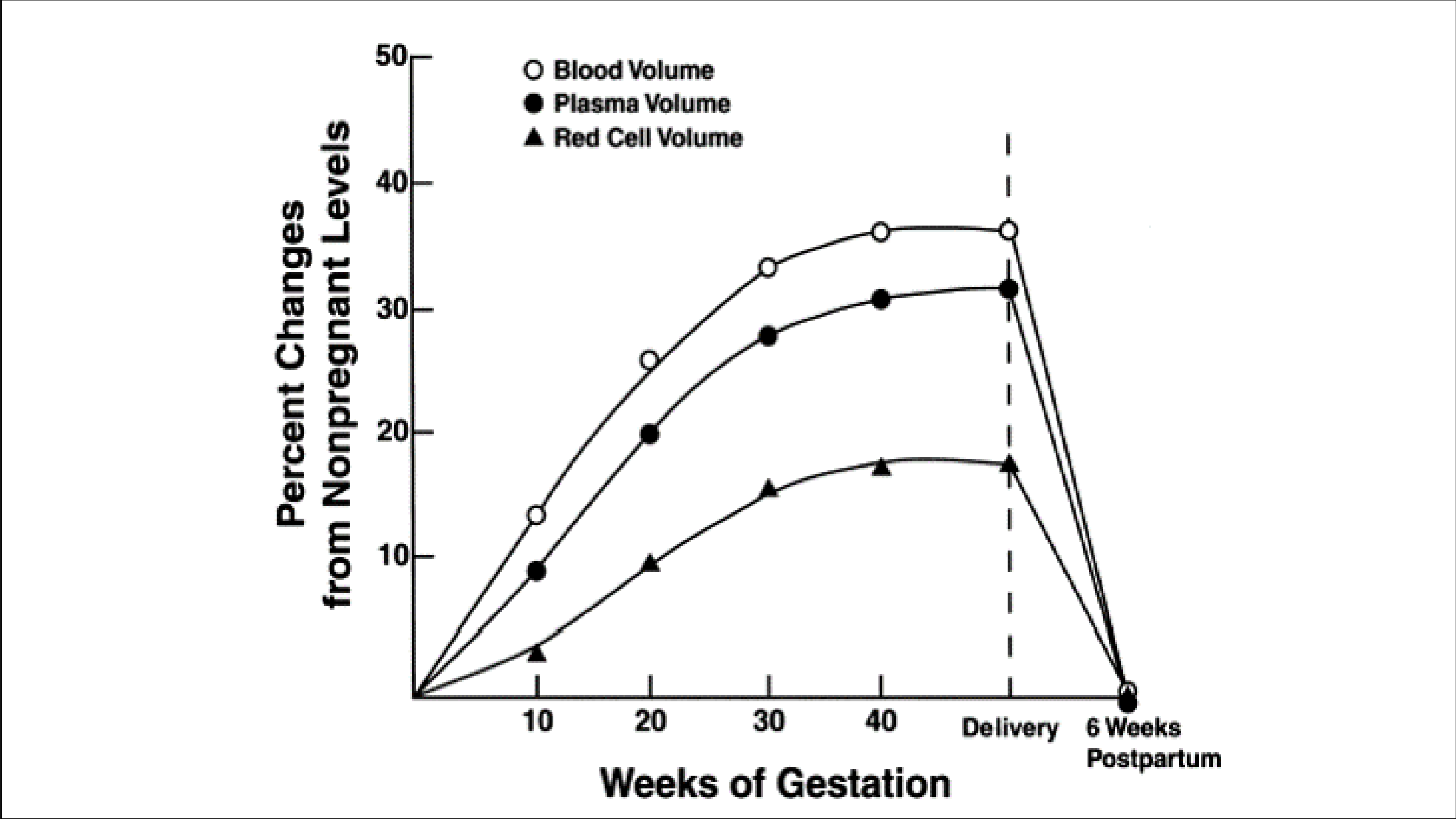
Plasma volume begins to expand within a few weeks of conception and may increase by up to 50% (1250ml) by the 34th week of conception. There is a gradual linear increase in red cell mass due to increased erythrocyte production resulting from increased erythropoietin.
The increase in RBC production found to be about 180ml (14%) without supplementary iron and 350ml (28%) when extra iron and folic acid are taken.
This relatively greater increase in blood volume results in a lower hematocrit.
Significance of physiological Changes:
- To meet the demands of the enlarged uterus with its greatly hypertrophied vascular system.
- To protect the mother, and in turn the fetus, against the deleterious effects of impaired venous return in the supine and erect positions.
- To safeguard the mother against the adverse effects of blood loss associated with parturition.
Etiology
Anemia in pregnancy most commonly results from a deficiency of Iron (in 95% of cases) or folate which could be as a result of:
- Reduced intake (nutritional).
- Increased demand like in cases of multiple pregnancy common in our area.
- Reduced storage as occurs with frequent poorly spaced conceptions and abnormal absorptions.
Malaria, endemic in the tropics, leads to combination of hemolysis, folate deficiency, and hypersplenism.
Chronic anemia from Hookworm infestation, HIV/AIDS, chronic infections (e.g. chronic pyelonephritis).
Hemolytic anemia from hemoglobinopathies, G6PD deficiency, autoimmune causes.
Signs and symptoms of anemia
Vague and nonspecific symptoms like tiredness, weakness, fainting attacks, breathlessness, swollen feet, headache, and pallor.
Jaundice and hepatosplenomegaly may be present in hemolytic anemia.
Signs of heart failure- tachycardia, palpitation, and raised jugular venous pressure etc. may be present in severe cases.
Other sign of Iron deficiency anemia include, headache, restless legs syndrome, and pica (in extreme situations).
Angular stomatitis, koilonychia and glossitis may be present in long standing iron deficiency anemia.
Aside from anaemia, folate and B12 deficiency are linked to neural tube defects.
Effects on pregnancy
Mild deficiency is linked to increased delivery bleeding, poor fetal iron stores and an increased placenta: fetus weight ratio, postpartum depression.
Severe maternal iron deficiency is associated with
- Intrauterine growth restriction
- Premature delivery
- Low birth weight
- Poor mental and psychomotor performance of babies
- Intrauterine fetal death
Investigations
To determine
- Degree or severity of anemia
- Type of anemia, and
- Possible causes
Basic investigations like packed cell volume (PCV) or/and hemoglobin concentration (Hb) will reveal the degree of anemia.
Full blood count (FBC) should be done if infection is suspected.
Measurement of other blood indices: MCV, MCHC, MCH, reticulocyte count, peripheral blood film smear will help to ascertain the type of anemia.
- MCV: Mean corpuscular volume- is increased in both B12 and Folic acid deficiency, and reduced in Iron deficiency.
- MCHC: Mean corpuscular hemoglobin concentration.
- MCH: Mean corpuscular Hemoglobin- High in Macrocytic and low in Microcytic anemia.
- Peripheral blood smear: Hypochromic Microcytic in Iron deficiency, Normochromic Macrocytic in folate deficiency
Thick and thin peripheral blood film for malaria parasite to rule out malaria infection.
Stools examined for ova, parasites and occult blood.
Genotype
Urinalysis- a clean catch mid-stream urine specimen, especially when there is suspicion of urinary infection
- Proteinuria, hematuria and nitrite may suggest UTI
- Bacteria colony count of over 105/ml is indicative of infection
- Bilirubin may suggest hemolytic condition.
Specific tests like serum levels of ferritin, Iron, Folate and Vitamin B12, total Iron Binding Capacity (TIBC).
note
Lab findings in Iron-deficiency Anemia
- Low PCV and Hb levels
- Reduced MCV, MCH AND MCHC levels (microcytosis, hypochromasia, anisocytosis/poikilocytosis)
- Low serum iron, ferritin, transferrin saturation percentage
- TIBC is elevated
Lab findings in Folic Acid Deficiency Anemia
- Low PCV and Hb levels
- Increased MCV, high MCH, normal MCHC
- Serum iron level is normal or elevated
- TIBC is low
- Serum folate is low
Prevention and Treatment
Oral ferrous and folic acid should be given daily to all pregnant women.
Malaria prophylaxis should be given.
Health education on child spacing and contraception, balanced diet, etc.
An average Western diet supplies around 250 μg/day of folate; however, requirements increase to around 400 μg/day during pregnancy, with deficiency most commonly due to lack of folate-rich vegetables.
Folate deficiency is more common in-
- Multiple pregnancy
- Frequent childbirth, and
- Adolescent mothers.
The body stores around 3 mg of B12, with a daily dietary requirement of 3μg /day. The only B12 source is animal foodstuffs; thus, vegetarians and vegans are most at risk of dietary deficiency.
Iron requirements increase due to expansion of red blood cell mass and rapid growth of the fetus. This need outstrips the 1 mg/d of iron available from the normal diet and must be met by supplementation of at least 40 mg/d of elemental iron (10% of which is absorbed). There is increased absorption in deficiency states.
WHO recommendations for pregnant women
- Daily oral iron and folic acid supplementation with 30 mg – 60 mg of elemental iron (a daily dose of 60 mg is preferred in areas where anemia is a public health problem like Nigeria).
- And 400 µg (0.4 mg) folic acid is recommended.
The equivalent of 65 mg of elemental iron is 200 mg ferrous sulphate, 180 mg ferrous fumarate or 500 mg of ferrous gluconate.
Folic acid should be commenced as early as possible (ideally before conception) to prevent neural tube defects.
In established cases: Ferrous sulphate 200mg (65mg of elemental iron) is given 3 times a day. If it is not well tolerated (there may be constipation, abdominal upset etc.)Ferrous fumarate or gluconate should be given.
Iron absorption is maximized when combined with ascorbic acid such as with fresh orange juice or a vitamin C preparation.
Parenteral therapy is useful in malabsorption and failed compliance, but otherwise does not produce a faster response than oral iron and side effects are common.
Proven folate deficiency anemia should be treated with folic acid (5 mg/day).
In all such cases of anemia, B12 deficiency must also be excluded, as folate may improve the anemia of B12 deficiency, but exacerbate any associated neurological deterioration.
In B12 deficiency, a single dose of 1000 μg of intramuscular B12 should lead to a reticulocyte response within 3–7 days.
Weekly injections should be employed until anemia resolves.
Severe anemia and moderate anemia close to/at term/ in labor requires transfusion with screened packed cells.
Introduction
Comprises of disorders affecting the structure, function and production of hemoglobin.
Hemoglobin is the oxygen binding protein of red blood cells and is a globular protein with quaternary structure.
Hemoglobin consists of four polypeptide subunits: 2 alpha chains (constant) and two beta, delta or gamma chains.
Hemoglobin A (HbA), also known as adult hemoglobin or α2β2. It consists of two alpha chains and two beta chains and is the most common human hemoglobin tetramer, comprising over 97% of the total red blood cell.
Hemoglobin A2 (HbA2) -normal variant of hemoglobin A. Consists of two alpha and two delta chains (α2δ2) and is found at low levels in normal human blood.
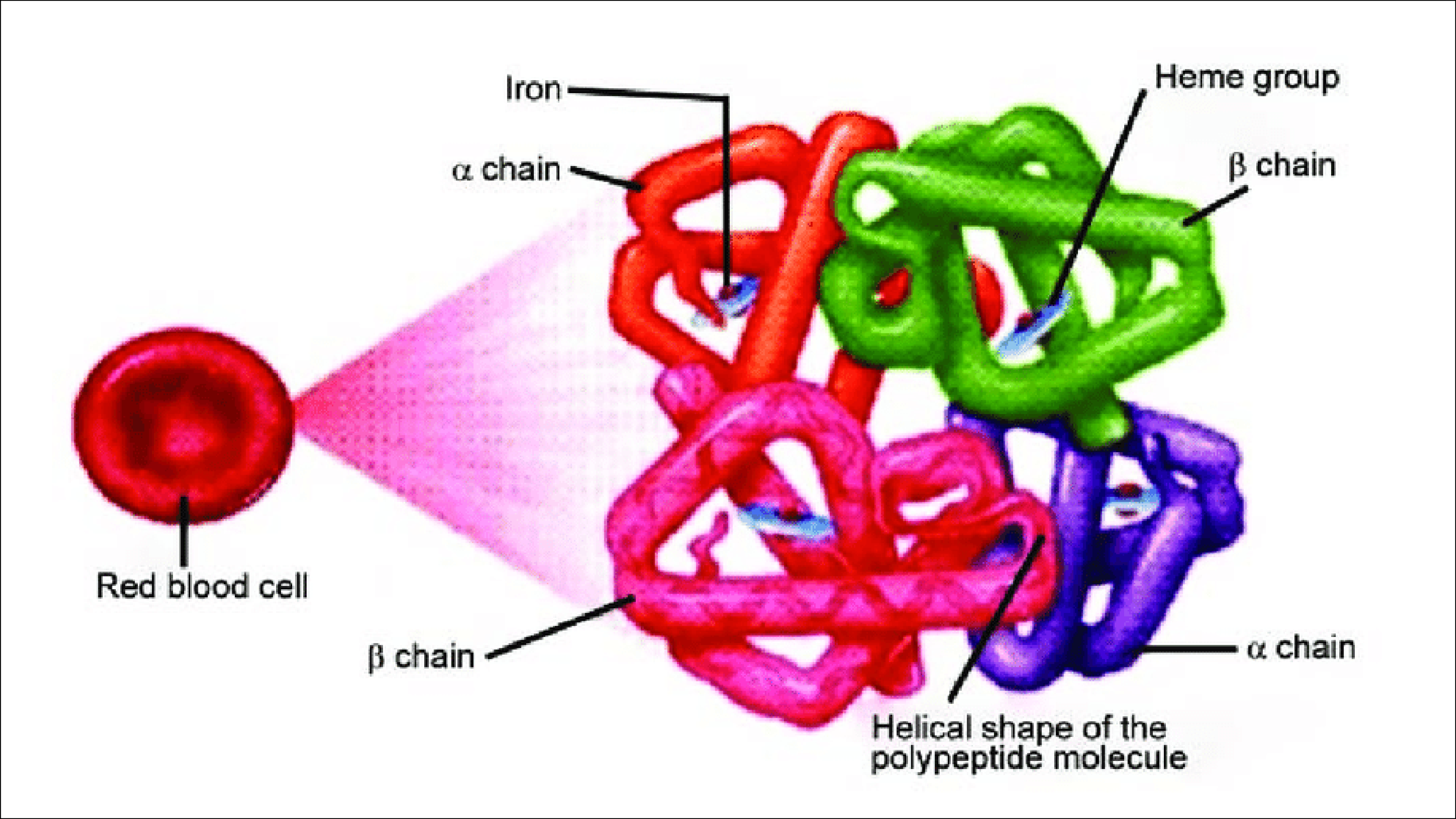
Hemoglobin F(HbF), or α2γ2 is the main oxygen transport protein in the human fetus during the last seven months of pregnancy and persists in the newborn until roughly 6 months old.
It is able to bind oxygen with greater affinity than the adult form, giving the developing fetus better access to oxygen from the mother's bloodstream.
Thalassemia disorders
Disorder of production of hemoglobin.
α-thalassemias are caused by an α-globin chain synthesis defect. They result from partial (α+) or total (α0) deletions. Total deletion or deficiency not compatible with life.
β-thalassemia syndromes are the result of insufficient (β+) or absent (β0) production of β-globin chains.
These are autosomal recessive condition.
Abnormal forms of hemoglobin
Include the Hb S, Hb C and Hb E.
In the HbS hemoglobin the β globin chain has an amino acid substitution of VALINE for GLUTAMIC ACID at CODON 6
While for Hb C, the β globin chain has substitution of LYSINE for GLUTAMIC ACID at CODON 6.
Hemoglobin E (HbE) is an abnormal hemoglobin with a single point mutation in the β chain. At POSITION 26 there is a change in the amino acid, from GLUTAMIC ACID to LYSINE. (Native to south East Asia).
Inherited as autosomal recessive disorders.
Homozygous – Hb SS, Hb CC, Hb EE.
Heterozygous –Hb SC, AS, SE.
The term “sickle-cell disease” includes all manifestations of abnormal HbS presentation. These include homozygous sickle-cell disease (HbSS) and a range of mixed heterozygous hemoglobinopathies (HbS/β-thalassemia, HbSC disease, and other combinations).
Sickle cell disease is prevalent in Tropical Africa, black Americans, offsprings of mixed marriages (black & white), Caribbean, Middle East and Mediterranean.
Incidence is about 5% of African stock for the trait and 1-2% are homozygous.
These are autosomal inherited diseases.
Decreased oxygen tension causes hemoglobin S to form insoluble polymer in curvilinear strands and these deform the normal biconcave shape of the erythrocytes (sickling).
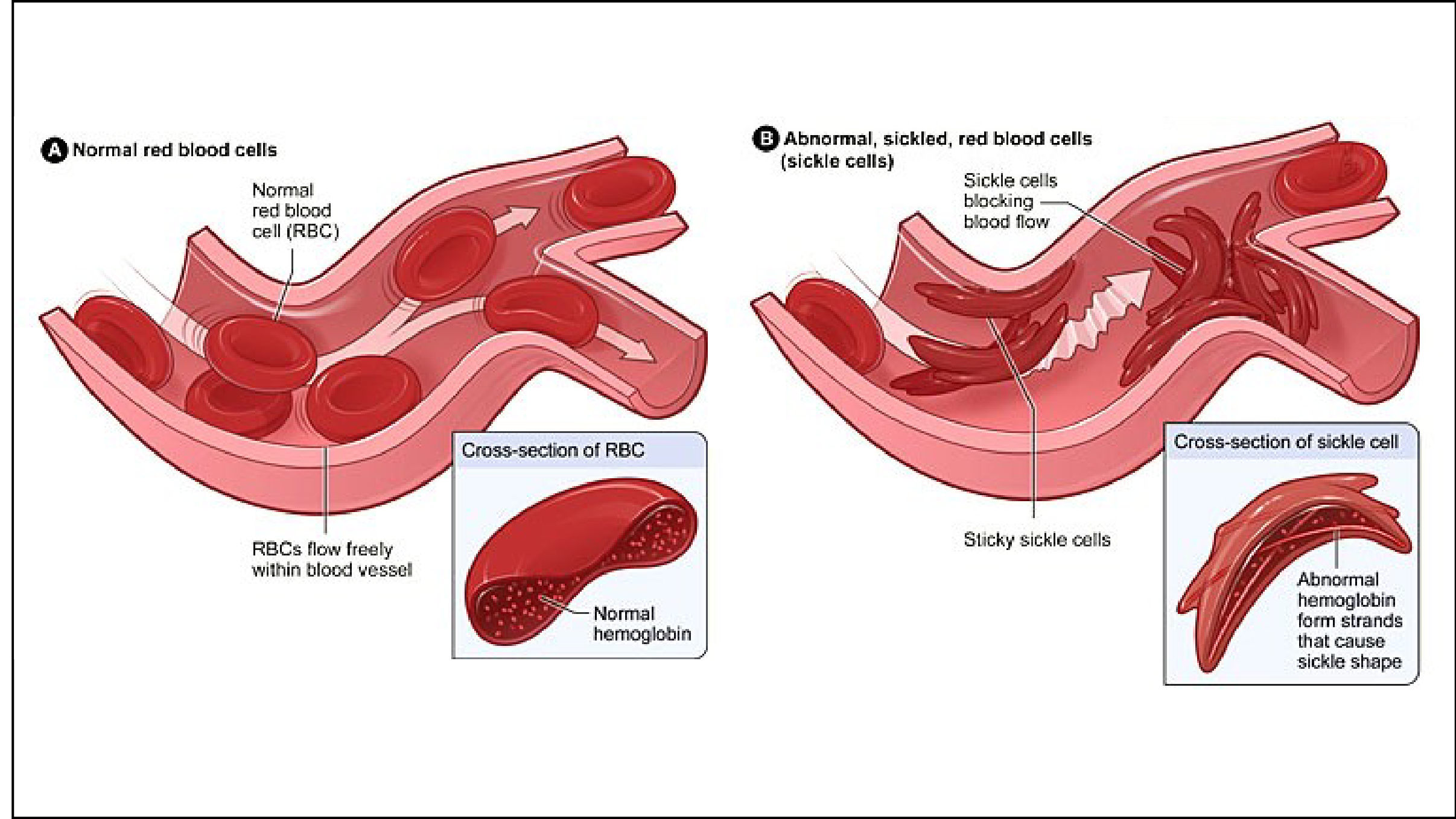
The sickle shape also results in altered motion through the microvasculature which can predispose the patient to vascular stasis, hypoxia, acidosis, and increased 2,3-diphosphoglycerate, which perpetuates the cycle by resulting in further deoxygenation and, thus, more sickling. The microvascular injury can result in ischemic necrosis and end-organ infarction.
These permanently damaged RBCs are then removed by the reticuloendothelial system, with the average RBC lifespan reduced to 17 days. The result is a chronic compensated anemia, with Hb typically measured between 6.5 and 9.5 g/dL.
Organs affected by chronic sickling include the spleen, lungs, kidneys, heart, and brain.
Patients with sickle cell anemia are functionally asplenic.
The spleen is crucial to the host response to infection by clearing polysaccharide-encapsulated bacteria. Encapsulated pathogens are opsonized with antibodies and then phagocytized by specialized macrophages in the spleen.
Therefore, immunization for and aggressive treatment against encapsulated organisms (pneumococcus and meningococcus) is recommended when such are diagnosed.
Hb SC is peculiarly associated with embolization of necrotic fat and cellular bone marrow with resultant respiratory insufficiency; aseptic necrosis of femoralhead and acute chest syndrome can result from the release of fat emboli after marrow infarction.
Clinical presentation
Presentation varies from a lifelong crippling hemolytic disorder (characterized by crises caused by infection, aplasia, infarction and hemolysis) to a diagnosis only made on a routine blood film examination. This variation may be due to the co-inheritance or persistence of fetal hemoglobin.
Chronic anemia: Results from shortened live span of RBC’s from intravascular trauma, hemolysis and phagocytosis in spleen.
Sickling of red blood cells: Leads to vaso-occlusion and infarction, Small vessels can be completely blocked causing Ischemia, pain, necrosis and organ damage e.g. avascular necrosis of head of Femur, chronic ulcers.
Crises: Pain, aplastic and sequestration.
Others: Increased susceptibility to infection, osteomyelitis, and respiratory infections.
Effects on pregnancy
High Risk pregnancy
Increased maternal mortality and morbidity. Increased risk of maternal infection (most often, pneumonia, UTIs, and endometritis), pregnancy-induced hypertension, heart failure, and pulmonary infarction. Anemia almost always becomes more severe as pregnancy progresses. Sickle cell trait increases the risk of UTIs but is not associated with severe pregnancy-related complications.
There is likely to be an increased risk of pre-eclampsia, early fetal wastage, stillbirths, preterm deliveries, fetal growth restriction and small-for-date baby, possibly through placental infarction.
Pseudotoxemia of pregnancy: characterized by systolic hypertension, proteinuria and severe bone pain. It is associated with bone marrow fat embolism and is highly fatal.
Investigations
Routine antenatal investigations.
Sickling test and hemoglobin electrophoresis.
Regular packed cell volume or Hb concentration.
Regular urine MCS.
Liver function tests where applicable.
X-rays where needed.
Treatment
Pre-conception care
Women and men with SCD should be encouraged to have the hemoglobinopathy status of their partner/partner to be determined before they embark on marriage or pregnancy
Genetic counselling
IVF with PGD (pre genetic diagnosis)
Prenatal diagnosis
Vaccination status should be determined and updated before pregnancy.
Antenatal care
Prevention of infections thro vaccinations e.g. polyvalent pneumococcal vaccine.
Routine antimalarial and folic acid.
Serial ultrasound.
Surveillance for asymptomatic bacteruria.
Regular checks on hematocrit level.
Prompt and aggressive treatment of infection and crises.
Management of labor
Admit into labor ward in the active phase > 4cm.
Maintain intravenous access and ensure adequate rehydration but avoid overloading.
Adequate analgesia should be given.
Nurse in left lateral position as much as possible.
Oxygen supplementation by face mask may be required.
Labour (Intrapartum management)
Open Partograph to record events during labor
Cardiotocographic monitoring of labor
Labor may be augmented or induced if the need arises
Shorten second stage of labor
Active management of 3rd stage
Prophylactic antibiotics should be given.
Caesarean section should be performed for obstetric indications.
Contraception
Encouraged to have small sized families with a maximum of 2 deliveries.
Contraceptive choice somewhat difficult
Oral contraceptive pills >> Thromboembolism
IUCD-Risk of infection esp. PID.
Progesterone based contraception encouraged e.g. depo, or implants.
Practice Questions
Check how well you grasp the concepts by answering the following questions...
- This content is not available yet.
Send your comments, corrections, explanations/clarifications and requests/suggestions